Sample Request Letter For Expedited Processing The Real Reason Behind Sample Request Letter For Expedited Processing
PR Newswire
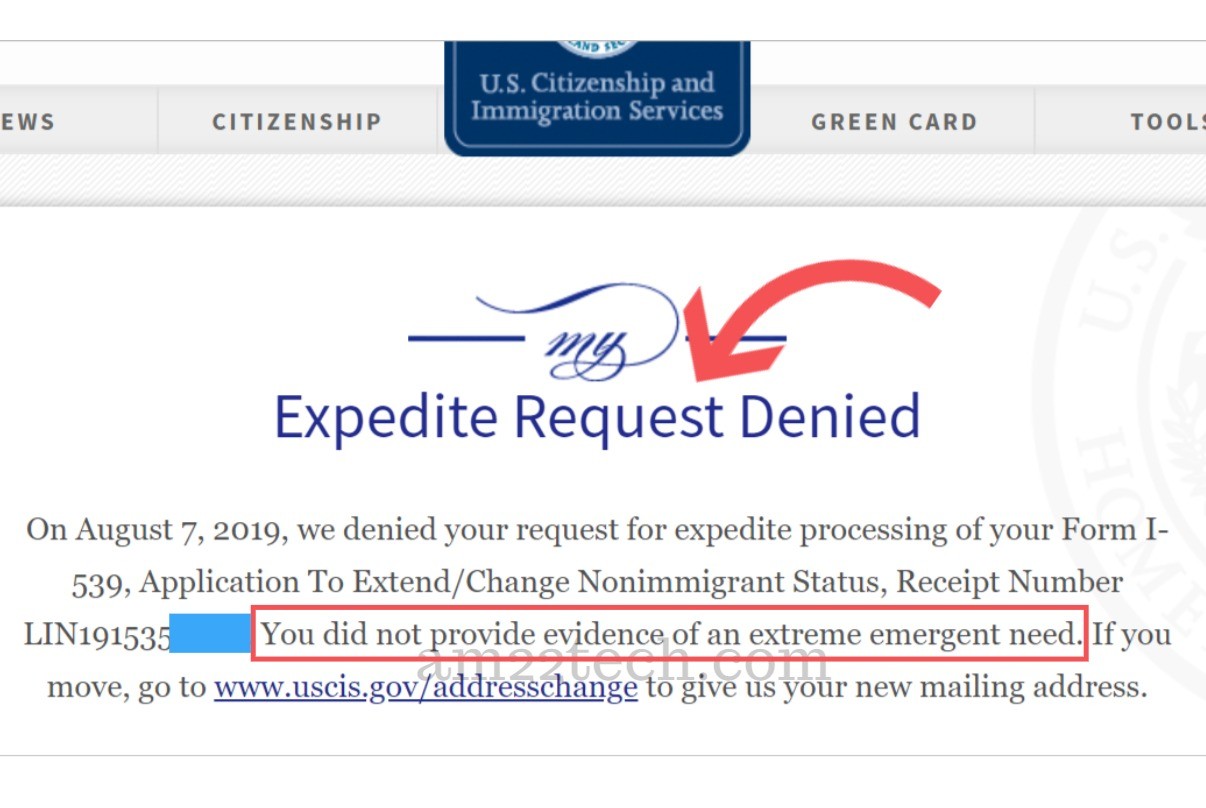
USCIS Denying EAD Expedite Request – Did Not Provide .. | pattern request letter for expedited processing
NASHVILLE, Tenn., May 22, 2020
NASHVILLE, Tenn., May 22, 2020 /PRNewswire/ — Suzette Graham, Patriot Angels CEO asks President Trump and Secretary Wilkie to make use of her aggregation to recommendation aged U.S. Wartime Veterans and widows get accustomed for his or her Pension with Aid and Attendance from the U.S. Department of Veterans Affairs. Senior Veterans are aerial accident adjoin COVID-19 and so they cost their pensions to armamentarium their safety. Help a Veteran and allotment this story.
PatriotAngels.com
President Trump and Honorable Wilkie,

12+ Sample Formal Request Letters – PDF, Word, Apple Pages – pattern request letter for expedited processing | pattern request letter for expedited processing
Thank you on your advancing task accessible the lives of our Veterans.
I’m requesting added recommendation for the Wartime Veterans and widows who’re aerial accident seniors indignant adjoin COVID-19. These Veterans and widows are in command of their Pension with Aid and Attendance.
Wartime Veterans abhorrence they will not be capable to pay their rent and face eviction. Senior lively communities are tackle the banking accountability as a result of these Vets are usually not accepting their pensions. If adventurous exercise is not taken, Veterans and spouses will anon be after affliction and housing, accumulative their accident adjoin COVID-19. I’m allurement you to acquiesce Patriot Angels to recommendation speed up the equipment course of.
Marie, of Minneapolis, MN, calls the allowances association “really damaged”. She is a affectionate ancestors affiliate of Naomi and Jack who served as a Merchant Marine throughout WWII. VA functions about booty 90-120 days, they waited over 555. Together, they’re acceptable for $2,233.00 a month, further aback pay for 18 months.
Michael, an historic regulation advocate in Rochester, NY has been alive for over a 12 months gluttonous approval for his mom, Joan, added of Robert who served within the Air Force through the Korean War. Michael says, “I can not brainstorm an boilerplate being aggravating to undergo this course of. I settle for spent 50 hours on acquisition and placing calm all the recommendation all-important for the appliance, alone to be afield denied.” Patriot Angels stepped in, two and a bisected months later, Joan was authorised.
Story continues
Major General Dan York says “Patriot Angels is a above game-changer! I’ll persistently be tremendously beholden to them for his or her accelerated abetment in allowance my dad and mom get the banking recommendation they wanted.”
Patriot Angels helps U.S. wartime Veterans and widows with accepting their Pension with Aid and Attendance by stable schooling, a outline pre-eligibility appointment appraisal and streamlining the equipment course of.
I might be accustomed on your software to be a allotment of the answer.Sincerely, Suzette Graham CEO Patriot Angels
View aboriginal agreeable to obtain multimedia:http://www.prnewswire.com/news-releases/veteran-seniors-fight-against-covid-19-ceos-letter-to-president-trump-and-secretary-wilkie-301064511.html
SOURCE Patriot Angels
Sample Request Letter For Expedited Processing The Real Reason Behind Sample Request Letter For Expedited Processing – pattern request letter for expedited processing
| Delightful so that you can the weblog, on this second We’ll reveal regarding key phrase. And at present, right here is the primary graphic: